Cure’nCaps helps you to design, develop and manufacture a specific delivery system which best meets the nature of your active ingredient, the considered administration route and therapeutic target.
Controlled drug delivery platforms, including microspheres, nanocapsules, liposomes and micellar systems, together with our specific know-how are provided to address various pharmacological issues for a wide range of active ingredients, from small chemical drugs to large biomolecules.
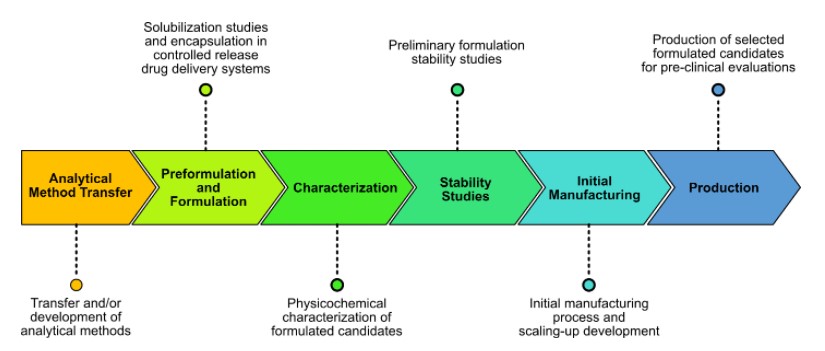
Cure’nCaps provides its unique expertise in formulation development from experimental design and lab-scale feasibility studies to batch production for pre-clinical evaluations. We also provide support in technology transfer to CMOs for upscaling and industrial GMP manufacturing.
Industrial objectives
For each project, Cure’nCaps takes industrial, economic and commercial requirements into consideration. Our expertise in project management allows rigorous development phase evaluation and control.
Following laboratory scale feasibility studies, Cure’nCaps manufacturing processes allow rapid determination and validation of transposition conditions for successful product manufacturing at industrial scales.
Formulation development services
- Drug nano and microencapsulation
- Drug stability improvement
- Drug solubility enhancement
- Targeted drug delivery (active and passive)
- Sustained release systems
- Hydro- and organogels
- Extrusion/Homogenization
- Solubility testing
- Fluorescent labeling
- Sterilization
- Lyophilization
- Batch production for preclinical evaluations
- Technology transfer to CMO/CDMO
Analytical Services
- Particle size analysis
- Zeta potential analysis
- Determination of drug loading
- Encapsulation efficiency
- Release assays
- Stability testing
- ELISA
- HPLC-UV analysis
- UV Spectrometry
- pH and Osmolarity
- Imaging
- Endotoxin and sterility assays